Next Generation Fast Charging Safe Battery
Battery is the key component of any of the electronic devices: Mobile Phones, Cameras, Laptops, Cars, Home Appliances and many others. Basically, we face three main challenges: as yet, batteries do not stay charged for a long time and need to be charged at a periodic interval. Charging time is another important factor, and most of the time we get bogged down by long charging time. This can be improved by making fast charging a possibility. Longer usage of a battery, used in a device brings in another factor to be considered: safety. Instability develops due to chemical change in a battery due to repeated charging / discharging and in a poorly designed battery; this could lead to fire hazard.
Basically, in Lithium-ion cells, lithium ions from the positive electrode (the cathode) are sent to the negative electrode (the anode) during charging. Lithium is stored in the electrode in the form of LiC6, one lithium atom is surrounded by six carbon atoms. By using silicon instead of carbon in anodes, lithium ions will combine with silicon to form Li15Si4. The 15-to-4 ratio means a smaller amount of anode material can store a lot more lithium, thus providing more energy storage.
Silicon-Lithium combine goes through a 300%-400% expansion and shrinkage in volume during charging and discharging. Over a period of time, this damages the battery and may create fire hazard.
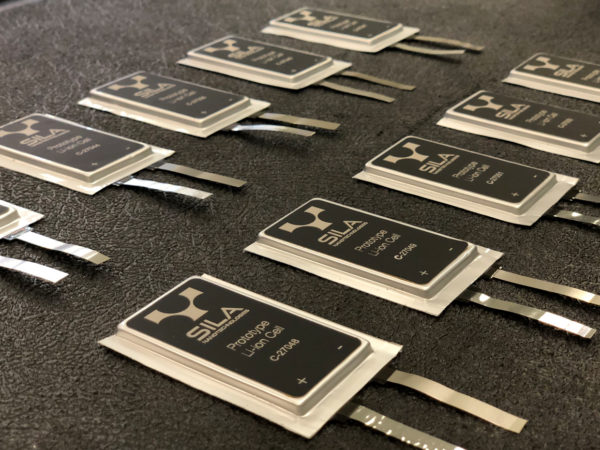
Solution: A California startup named Sila Nanotechnologies plans to commercialize a silicon-rich anode material, company cofounder and Georgia Tech Professor Gleb Yushin narrates that Sila has developed new anode material which is composed of particles that are similar in size to the present graphite anodes, but they contain silicon inside a porous scaffolding, which provides room for the silicon to expand and contract without coming into contact with the electrolyte. This allows batteries made with this silicon-rich anode material to perform well for 400 to 1,000 full charge-discharge cycles, which is more than enough for most applications.
Depending on the application, use of this anode material will boost battery capacity initially by about 20-40 percent better while allowing the anode to be reduced in thickness by up to 67 percent, thus permitting the battery to be charged as much as nine times as fast. It will also suppress the formation of threadlike metallic dendrites, which can cause cells to short out internally and burst into flame.
This success is bringing BMW to co-work with Sila to actually use this new anode in its electric cars. Professor Yushin expects the price of battery to be comparatively low, thus its usage will be heavy in millions of devices.
Another company from California, Enovix, is being led by Ashok Lahiri, cofounder and chief technology officer for Enovix, and his two colleagues. They were planning to use solar-grade silicon as they are going to use an anode that is made entirely of silicon and silicon oxides. But they realized that in reality, solar-grade substrates could not scale. Enovix is now using a metal foil instead of a silicon wafer as the substrate for its battery, keeping the overall geometry of the battery, the same. By keeping the anode stack under high pressure, Enovix process inhibits the expansion during charge and allows the anode to be made entirely from silicon and silicon oxides. Lahiri projects that an efficiency of 30% to 70% will be achievable, depending on the application.
Another major research promise comes from the University of Texas at Austin, from a team led by John Goodenough, co-inventor of the now ubiquitous lithium-ion battery and emeritus professor at the Cockrell School of Engineering at the University of Texas, Austin. Maria Helena Braga, a visiting research fellow at UT Austin and engineering professor at the University of Porto in Portugal, is the co-developer of this project.
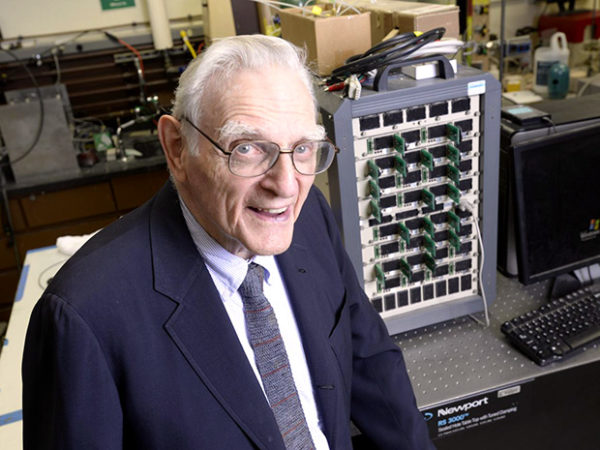
John Goodenough, co-inventor of the lithium-ion battery, leads a team of researchers developing the glass battery.
The new battery technology uses a form of glass, doped with reactive “alkali” metals like lithium or sodium, as the battery’s electrolyte (the medium between cathode and electrode that ions travel across when the battery charges and discharges). As outlined in a research paper and recent patent filing (of which Goodenough, 94, says more are forthcoming), the lithium- or sodium-doped glass electrolyte offers a new medium for novel battery chemistry and physics. This process is still under development but promises magical properties.
- The lithium- or sodium-glass battery has three times the energy storage capacity of a comparable lithium-ion battery. But its electrolyte is neither flammable nor volatile, and it does not appear to build up the spiky “dendrites” that have plagued lithium-ions as they charge and discharge repeatedly and can ultimately short out, causing battery fires. So, if the glass batteries can be scaled up commercially, which remains uncertain in this still-proof-of-concept-phase research, the frightening phenomenon of flaming or exploding laptops, smartphones, or EVs could be a thing of the past.
- The glass battery charges in “minutes rather than hours.” Because the lithium- or sodium-doped glass endows the battery with a far greater capacity to store energy in the electric field. So, the battery can, in this sense, behave a little more like a lightning-fast super-capacitor. (In technical terms, the battery’s glass electrolyte endows it with a higher so-called dielectric constant than the volatile organic liquid electrolyte in a lithium-ion battery.)
- Early tests of their technology suggest it is also capable of perhaps thousands of charge-discharge cycles, and could perform well in both extremely cold and hot weather. (Initial estimates place its operating range between below -20º C and 60º C.) And if they can switch the battery’s ionic messenger atom from lithium to sodium, the researchers could even source the batteries more reliably and sustainably. Rather than turning to controversial mining operations in a few South American countries for lithium, they will be able to source sodium in essentially limitless supply from the world’s seawater.
- MIT’s Prof. Donald Sadoway says he is eager to learn more about the technology as it continues to be developed. In particular, he is paying attention not so much to how quickly the battery charges but how well it can retain its energy. “The issue is not can you do something at a high charge rate,” he says. “My big question is about capacity fade and service lifetime.”
- Safety issues: Sadoway adds, perhaps the chief innovation behind Goodenough and Braga’s technology is the possibility that they have solved the flaming and exploding battery problem.
If Goodenough, Braga, and collaborators can ramp up their technology, there would clearly be plenty of upsides. Goodenough says the team’s anode and electrolyte are more or less ready for prime time. But they’re still figuring out if and how they can make a cathode that will bring the promise of their technology to the commercial marketplace.