For Light-Emitting Diodes, Size Matters
LED’s were a huge break-through from traditional bulbs like incandescent and fluorescent. They rank supreme in terms of lighting efficiency, durability, and lifetime. Although light-emitting diodes don’t have a heat-dissipating, energy-wasting burning filament, they do have just a tiny plastic enclosure containing photon emissions. That’s a pretty nice trade-off.
Just for Christmas lights alone, LED gives the shatter-free guarantee and a lower December utility bill—happy New Year, right? But that’s not all. These diodes have a lifetime of 50,000 hours or more, so even if the lights are left on 365 days a year, they’ll still last for 5-6 years. And this goes for every other lighting application that utilizes LED technology.
Though it looks like LED should replace all lighting applications already, traditional diodes are not without problems. Due to the nature of tvs semiconductor, at high voltages LED’s are no longer as efficient in converting electricity into light; i.e. the current begins increasing linearly with voltage instead of exponentially due to the resistance of the metal, semiconductor, or wiring of the diode. This has been especially problematic for scientists trying to get the diodes to emit high frequency colors like green, as the required energy to do so is beyond traditional LED’s voltage capacity.
Yet in order for light-emitting diodes to truly dominate real-world applications from lighting up our homes to flatscreen TV’s, smartphones, etc., the technology needs to be able to self-emit the entire range of RBG to produce all the colors in between, especially white light. The dilemma then is how can we obtain high-frequency emissions if cranking up the power is not an option?
Answer: Give the electrons a nanoscale playground.
Nanowire LED
Researchers at the University of Michigan found out getting a complete set of LED colors is actually a simple process at the atomic level. One of the researchers, Emmanouil Kioupakis explains the theory:
“If you reduce the dimensions of a material to be about as wide as the atoms that make it up, then you get quantum confinement. The electrons are squeezed into a small region of space, increasing the bandgap energy.”
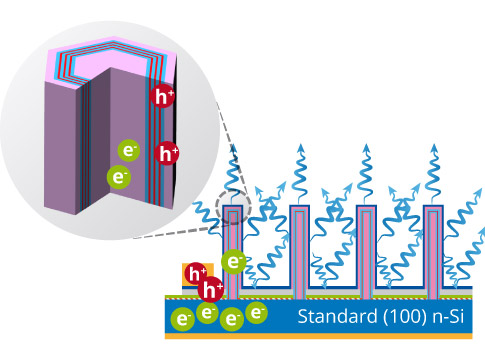
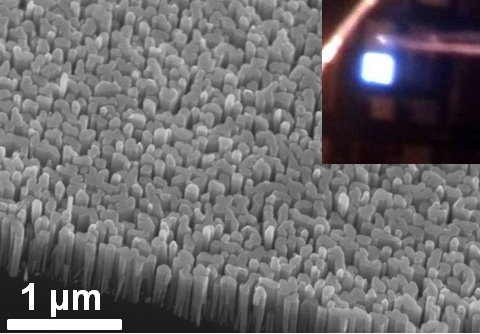
A bit of background: nanowire LED’s are typically ”grown” using molecular beam epitaxy (MBE), through which tiny crystals are deposited directly onto bare silicon. Like traditional LED’s, there are electrons and a bandgap they must cross in order to emit light, only now researchers are only giving them atom-sized vertical space to move about, resulting in a diode with a diameter of 100 nanometers, about 500 times smaller than the human hair.
Aledia
So far these scientists have been successful in growing such nitride nanostructures to test if they can a better spectrum while maintaining conversion efficiency, and the results were affirmative. In their simulation, they were able to get the desired bandgap increase which could produce higher frequency lights like green. No, people won’t easily detect one of these LED wires even with a hard squinting, but bunch a whole lot of them together and what’ll result is a 3D vertical depiction with real, loss-less colors we can see.
nanotechweb
Dr. Kioupakis states that with nanotechnology, it’s now possible to get any color to come through the light-emitting diodes simply by tailoring the width of the wire in the nanoscale range. And beyond high-definition colors, giving electrons less vertical room to “wander” results in a higher electron-to-photon efficiency as well. Dr. Emmanouil Kioupakis explains how this works:
“When the two materials don’t have the same spacing between their atoms and you grow one over the other, it strains the structure, which moves the holes and electrons further apart, making them less likely to recombine and emit light.
In a nanowire made of a single material, you don’t have this mismatch and so you can get better efficiency. Bringing the electrons and holes closer together in the nanostructure increases their mutual attraction and increases the probability that they will recombine and emit light.”
For sure, the ultra-efficient properties of nanowire LED’s will do well with energy-saving appliances and battery-dependent devices. Here’s a case where less is more, because for consumers, these much, much smaller LED’s will bring much, much more colors, efficiency, and out-of-the-pocket savings.
Source: Nano Letters―Visible-Wavelength Polarized Light Emission with Small-Diameter InN Nanowires