What Is Genotoxicity Screening?
Genotoxicity screening Is scientific research testing of new chemical compounds to determine their safety. It is widely used by companies such as Gentronix throughout drug development to assess a product’s ability to cause mutations and damage to genetic material and to identify the possibility that these can lead to adverse effects such as cancer.
What is it?
Not all genotoxic substances are mutagenic, yet genotoxicity can lead to direct or indirect effects on the DNA, including aberrant cell division, mutation induction and direct DNA damage. Genotoxins can include radiation and chemical substances, which depending on their impact on organisms, can be carcinogens, mutagens and teratogens, and genotoxicity screening helps to begin identifying any potential liabilities early.
Decisions can then be made early on whether a project should be abandoned, requires further mechanistic testing or could be continued in different conditions (such as more restricted use). With the ability to carry out testing in bacterial, mammalian and yeast cells, genotoxicity screening results can be conducted without using animals.
Why is genotoxicity screening needed?
Testing the potential for adverse effects of new drugs, agricultural chemicals and some food products is necessary to ensure public safety. Genotoxicity testing is also widely used in cosmetic, perfume and personal care industries and now based solely on non-animal data.
Rigorous assay testing allows manufacturers to build a complete picture of a substance’s potential to cause harm. Its genotoxic potential can be identified, and where necessary further testing or investigations can be carried out to identify frameshifts and point mutations.
What can it achieve?
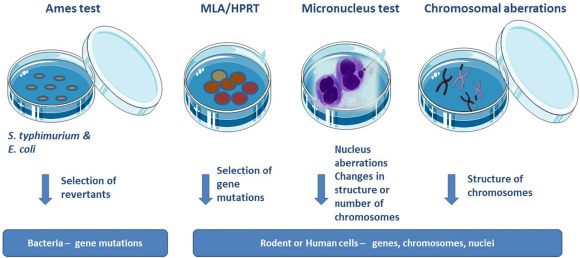
Through Ames testing, mouse lymphoma and in vitro micronucleus studies, regulatory genotoxicity studies enable developers to demonstrate chemical safety and show that regulatory requirements have been addressed. Predictive genotoxicity screening is used early in the research and development phase of drug creation before any final product’s regulatory tests are necessary.
Predictive genotoxicity assays enable accurate, rapid low compound screening for early identification of any issues. Early identification reduces development costs and changes needed to be identified early and provides knowledge about future viability. Some projects that previously faced being discarded can now benefit from further testing to determine at what point or level an issue becomes damaging or critical and provide for a calculated risk or limit to be set to determine acceptable risk.
An early view of toxicological hazard will help identify potential challenges early in the project cycle, allowing for early decisions to be taken on a project’s future.
If the initial genotoxicity testing results show potential issues, it is then possible to carry out further mechanistic testing to assist with managing identified hazards. Understanding the biological relevance of positive results can allow the mechanism of such changes to be identified and a risk assessment based on exposure to be made.
Why a positive doesn’t mean your product should be abandoned
Positive results through genetic toxicology testing no longer always need to signal the end of a project. Understanding the biological relevance of a positive result can, when the toxicological mechanism and mode of action is identified, enable a risk assessment based on exposure to be made. This has seen many projects go to a successful conclusion once levels and exposure risks were identified and removed or accounted for.