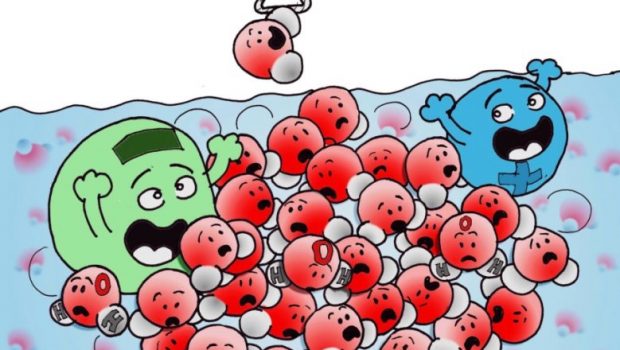
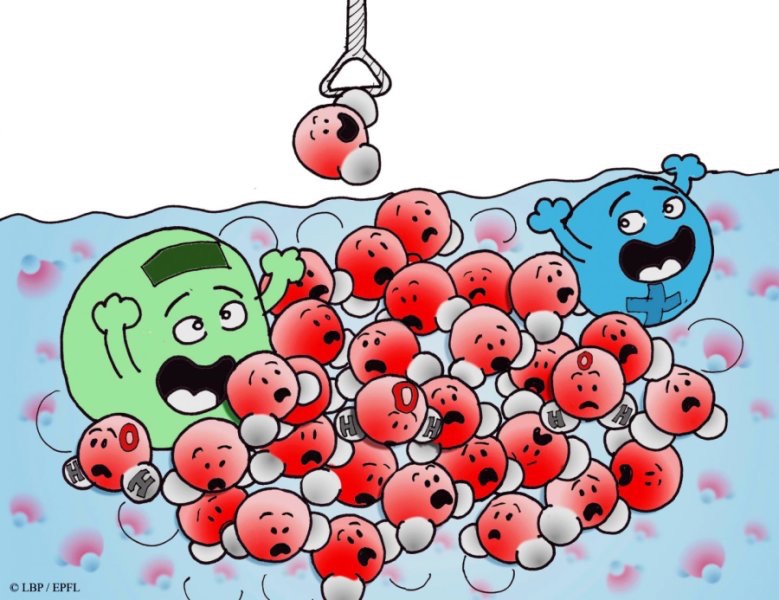
One ion impacts millions of water molecules
Water makes up more than half of our body content and planet, and even then, our understanding about its unique properties and behavior are constantly evolving. It was once thought that a single ion like salt has the ability to affect 100 water molecules. A new study published by a research institute in Switzerland now shows that number is more like a million.
Rearranged water molecules
Water molecules are held together by hydrogen bonds that continuously form and break. The collective hydrogen-bond interactions are responsible for water’s high melting and boiling points, large viscosity, and high surface tension, but it doesn’t take much to alter them.
Using ultra-sensitive optical equipment, researchers at Ecole Polytechnique Fédérale de Lausanne’s Laboratory for Fundamental BioPhotonics observed a single salt ion twist the bonds of several million water molecules within nanometers range, making them line up a certain way. They used an optical fs-ESHS method leveraging water’s light scattering properties to detect which and how many molecules displayed similar orientations.
“Orientationally-correlated water molecules scatter light of the double frequency of the incoming pulses. Water molecules that are not correlated in their orientation do not scatter this light,” said Sylvie Roke, Julia Jacobi Chair in Photomedicine who led the research group. To ensure the generated light pulse doesn’t interfere with the water’s structure, lasers are chosen such that its wavelength doesn’t match with the energy levels of the molecules. This makes the water transparent to the incoming laser pulse and also to the generated light, added Dr. Roke.
One thing they found was that the vast number of reoriented molecules made water “stiffer”.
“The ions make it a little bit harder for the water molecules to rotate away when they form a new hydrogen bond with another water molecule,” explained Dr. Roke, “this type of reorientation happens a million times in a millionth of a second.”
In another test, the group dipped a thin metal plate into ion-infused water and observed an unexplained phenomenon in water research – that ions reduce the surface resistance of water, and having a few ions in the liquid made it easier to pull the metal out.
Of course, not all water molecules are equal. Dr. Roke’s team found that in the case of heavy water whose molecules come with an extra neutron, it took a higher concentration of ions to produce the stiffness and higher surface tension previously observed.
The EPFL group has stated that its findings are not meant to explain some phenomena like water memory. The authors do note that “every new piece of knowledge gives greater insight into how life works”, and there is still much to uncover about water’s mysterious properties.